Ever wondered why the Incredible Hulk turned green after being exposed to gamma radiation? Okay, maybe that’s just comic book fiction, but gamma rays are a real and powerful force of nature. They’re a form of electromagnetic radiation, the highest energy kind, and they’re notoriously difficult to block. So, why is gamma radiation so hard to stop? It boils down to their unique properties and how they interact with matter.
Let’s start with the basics. Gamma radiation is like the heavyweight champion of the electromagnetic spectrum. It’s emitted during radioactive decay, nuclear reactions, and other high-energy processes. Think of it as the super-charged sibling of X-rays. While both are electromagnetic radiation, gamma rays pack a much bigger punch, carrying significantly more energy. This extra energy is precisely what makes them so penetrating and, consequently, so hard to stop. Unlike alpha particles, which are relatively heavy and carry a charge, or beta particles, which are lighter and also charged, gamma rays are massless and have no charge. This lack of charge means they don’t readily interact with the electrical forces within atoms, allowing them to slip through materials that would easily block other types of radiation. They’re like the ninjas of the radiation world – silent, unseen, and incredibly difficult to defend against.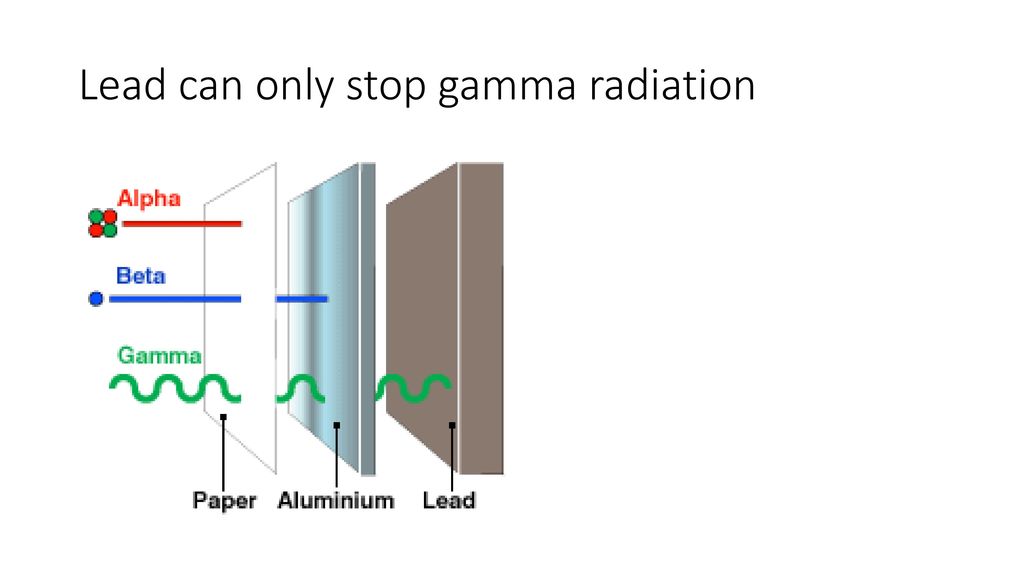
What Makes Gamma Rays So Penetrating?
So, we’ve established that gamma rays are high-energy photons, but what does that really mean? Let’s break it down. Think of the electromagnetic spectrum as a vast highway, with different types of radiation occupying different lanes. Radio waves are cruising in the slow lane, microwaves are a bit faster, visible light is zipping along, and then you have X-rays picking up speed. But gamma rays? They’re in the fast lane, flooring it at the speed of light, with the highest energy of them all. This high energy is the key to their penetrating power. It’s like a bowling ball (gamma ray) versus a ping pong ball (alpha particle) – the bowling ball is going to plow through much more stuff.
Gamma Radiation: A Deep Dive
Gamma rays are pure energy, unlike alpha and beta particles, which are actual particles with mass and charge. This lack of mass and charge is crucial. Alpha particles, being positively charged and relatively heavy, are like clumsy sumo wrestlers. They bump into things easily and are quickly stopped by even a sheet of paper. Beta particles, being lighter and negatively charged, are a bit more agile, but they still interact with the electromagnetic forces within atoms and can be stopped by a thin sheet of aluminum. But gamma rays? They’re like ghosts, slipping through the atomic defenses with ease.
Key Properties of Gamma That Make Shielding Difficult
Several key properties contribute to the challenge of shielding gamma radiation:
- High Energy: As mentioned before, this is the primary culprit. The higher the energy, the more penetrating the gamma ray.
- No Mass or Charge: This lack of interaction with electromagnetic forces makes them incredibly difficult to stop. They don’t get “stuck” like charged particles do.
- Speed of Light: Their incredible speed further contributes to their penetrating power. They zip through materials before the material has a chance to really react.
The Complex Interaction of Gamma with Matter
While gamma rays don’t interact with matter as readily as other forms of radiation, they do interact, just in different ways. There are three primary interaction mechanisms:
- Photoelectric Effect: This is more common with lower-energy gamma rays. The gamma ray interacts with an atom, ejecting an electron. Think of it like a billiard ball hitting another ball and knocking it off the table.
- Compton Scattering: This is the dominant interaction for gamma rays in the energy range we usually encounter. The gamma ray collides with an electron, losing some energy and changing direction (scattering). It’s like the billiard ball glancing off another ball, changing its trajectory. This scattering effect is a major reason why shielding is so challenging. The gamma ray isn’t absorbed; it just changes direction and keeps going.
- Pair Production: This occurs with very high-energy gamma rays. The gamma ray interacts with the nucleus of an atom and transforms into an electron-positron pair. This requires a lot of energy, and it’s less common in typical shielding scenarios.
These interactions, particularly Compton scattering, explain why gamma is hard to stop. The gamma ray doesn’t just get absorbed; it scatters, potentially changing direction and penetrating further into the shielding material. This means that simply having something in the way isn’t enough; you need enough of something to increase the odds of these interactions happening and, hopefully, the gamma ray losing enough energy to be considered stopped.

The Challenge of Shielding Gamma Radiation: Why Is Gamma Hard To Stop? The Shielding Challenge
So, now we understand why gamma is hard to stop on a fundamental level. Its high energy, lack of charge, and interactions with matter make shielding a real challenge. It’s not like building a brick wall to stop a runaway car; it’s more like trying to catch smoke with a net.
Gamma’s Incredible Penetration Power: Why it’s Hard to Stop
Gamma rays are notorious for their ability to penetrate materials that would easily stop other types of radiation. Alpha particles, for instance, can be stopped by a sheet of paper, while beta particles require a thin sheet of aluminum. Gamma rays, however, can easily pass through these materials and even thicker barriers like wood or concrete. Imagine trying to stop a bullet with a tissue – that’s what trying to stop gamma rays with inadequate shielding is like.
| Radiation Type | Penetration Power |
|---|---|
| Alpha | Low (stopped by paper) |
| Beta | Medium (stopped by thin aluminum) |
| Gamma | High (requires dense materials like lead or thick concrete) |
Ionization and Biological Effects: Why Stopping Gamma is Important
Beyond just being hard to stop, gamma radiation is also dangerous. It’s an ionizing radiation, meaning it carries enough energy to knock electrons off atoms and molecules. This ionization can damage DNA and other cellular components, potentially leading to health problems like cancer. This is why gamma is hard to stop and why it’s so important to do so. It’s not just about blocking a beam of energy; it’s about protecting living tissue from potentially harmful interactions.
Why Simple Barriers Aren’t Enough to Stop Gamma
The challenge with gamma shielding isn’t just about putting something in the way; it’s about putting enough of the right material in the way. Thin barriers, even of dense materials, are often ineffective because of Compton scattering. The gamma ray might interact, change direction, and continue its journey. This is where the concept of the “half-value layer” comes in. The half-value layer is the thickness of a material required to reduce the intensity of gamma radiation by half. This means that to reduce the intensity significantly, you need multiple half-value layers of material. This is why gamma shielding often involves thick and dense materials. It’s not enough to just slow the gamma ray down a little; you need to stop it entirely, or at least reduce its energy to a safe level.

Effective Gamma Shielding Techniques: How to Stop Gamma Radiation
Now that we understand the challenges, let’s talk about solutions. How do we stop gamma radiation? It’s a matter of understanding how gamma rays interact with matter and using that knowledge to our advantage.
The Role of Density in Gamma Shielding
Density is king when it comes to gamma shielding. Denser materials have more atoms packed into a given space, increasing the probability of a gamma ray interacting and losing energy. Think of it like trying to stop a swarm of bees. A thin net might let some through, but a dense, thick net is much more effective. Similarly, dense materials like lead and concrete are much better at stopping gamma rays than less dense materials like wood or plastic.
The Importance of Shielding Thickness for Stopping Gamma
Density isn’t the only factor; thickness also plays a crucial role. Even a dense material won’t be effective if it’s too thin. The thicker the shielding, the more opportunities the gamma ray has to interact and lose energy. It’s like having a thicker net for those bees – the more layers, the better the protection. The required thickness depends on the energy of the gamma rays and the intensity of the source. Higher energy gamma rays require thicker shielding.
Exploring Materials That Effectively Block Gamma
Several materials are commonly used for gamma shielding, each with its own advantages and disadvantages:
- Lead: Lead is a classic gamma shielding material due to its high density. It’s relatively easy to work with and is often used in medical and industrial applications. However, lead is also toxic, so it needs to be handled carefully.
- Concrete: Concrete is another common choice, especially for large-scale shielding like in nuclear power plants. It’s not as dense as lead, but it’s much cheaper and can be poured into various shapes. The effectiveness of concrete shielding depends on its density, which can be adjusted by adding different aggregates.
- Depleted Uranium: Depleted uranium is incredibly dense and is used in specialized applications where space is limited, such as in some portable radiation sources. However, it is also radioactive, although significantly less so than naturally occurring uranium, and requires careful handling and disposal.
- Tungsten Alloys: Tungsten alloys offer a good balance of density and workability. They are often used in situations where lead is impractical, such as in medical applications.
Designing Effective Gamma Shields: What You Need to Know
Designing effective gamma shielding is a complex process that requires careful consideration of several factors:
- Source Strength: The stronger the gamma source, the more shielding you’ll need. A small sample of radioactive material will require less shielding than a large one.
- Energy of the Gamma Rays: Higher energy gamma rays are more penetrating and require thicker shielding.
- Geometry of the Shield: The shape and configuration of the shield can also affect its effectiveness. For example, a spherical shield might be more effective than a flat one for a point source of gamma radiation. You need to consider where the radiation is coming from and ensure the shielding completely surrounds it.
It’s not just about throwing a bunch of lead or concrete at the problem. Effective shielding design requires careful calculations and consideration of all these factors to ensure adequate protection.

Practical Applications of Gamma Shielding: Where Do We Use Gamma Shielding?
Gamma shielding isn’t just a theoretical concept; it has numerous real-world applications, protecting us from the potentially harmful effects of gamma radiation in various fields.
Gamma Shielding in Medicine
Gamma radiation is used in both diagnostic and therapeutic applications in medicine, and shielding plays a vital role in ensuring patient and staff safety.
- Radiation Therapy: In radiation therapy, high-energy gamma rays are used to target and destroy cancerous cells. Precise shielding is crucial to protect healthy tissues from the radiation beam. Linear accelerators, which generate the gamma rays, are heavily shielded with lead and concrete to contain the radiation and direct it precisely at the tumor.
- Diagnostic Imaging: Techniques like PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography) scans involve the use of radioactive isotopes that emit gamma rays. Shielding is used in the design of the scanners themselves and in the handling and storage of the radioactive materials. This ensures that medical personnel and patients are exposed to minimal radiation.
Gamma Shielding in the Nuclear Industry
The nuclear industry is perhaps the most prominent user of gamma shielding, given the high levels of radioactivity involved.
- Nuclear Power Plants: Nuclear power plants utilize massive amounts of shielding, primarily concrete, to protect workers and the public from the intense radiation produced in the reactor core. The reactor itself is encased in thick layers of concrete and steel, and spent fuel rods are stored in specially designed pools or casks with thick shielding.
- Waste Storage: Radioactive waste from nuclear reactors and other sources emits gamma radiation and requires careful handling and storage. Waste is often stored in heavily shielded containers, and long-term storage facilities are designed with thick concrete and other shielding materials to prevent radiation leakage.
Gamma Shielding in Research Settings
Gamma radiation is also used in various research applications, and shielding is essential to protect researchers and lab personnel.
- Particle Accelerators: Particle accelerators, used to study the fundamental building blocks of matter, generate high-energy particles that can produce gamma radiation. These facilities require extensive shielding, often using a combination of concrete, lead, and other materials, to contain the radiation.
- Radioisotope Handling: Research labs often use radioisotopes for various experiments. These isotopes emit radiation, including gamma rays, and must be handled and stored in shielded containers to minimize exposure. Regulations are in place to ensure the safe handling and storage of these materials.
It’s important to note that even in these controlled environments, the principles of why gamma is hard to stop are always at play. Shielding design must account for the specific isotopes being used, their activity levels, and the energies of the emitted gamma rays. There’s no one-size-fits-all solution; each situation requires careful evaluation and tailored shielding design.

Why Is Gamma Hard to Stop? A Recap and Key Takeaways
So, we’ve journeyed through the world of gamma radiation, exploring its unique properties and the challenges it presents for shielding. Let’s recap the key takeaways:
- Why is gamma hard to stop? It boils down to its high energy, lack of mass and charge, and its interaction mechanisms with matter, particularly Compton scattering. These factors allow it to penetrate materials that would easily block other types of radiation.
- Gamma radiation is a form of electromagnetic radiation, the highest energy form, and is emitted during radioactive decay and other nuclear processes.
- While gamma rays can be challenging to shield, it’s not impossible. Effective shielding relies on using dense materials like lead and concrete, and ensuring sufficient thickness to maximize the probability of gamma ray interactions.
- The concept of the half-value layer illustrates how increasing the thickness of the shielding material reduces the intensity of the gamma radiation.
- Shielding design must consider the source strength, the energy of the gamma rays, and the geometry of the shield to ensure adequate protection.
- Gamma shielding plays a crucial role in various applications, including medicine, the nuclear industry, and research settings, protecting people from the potentially harmful effects of gamma radiation.
While gamma radiation might seem intimidating, understanding its properties allows us to develop effective shielding strategies. It’s a testament to human ingenuity that we can harness and control this powerful force of nature, using it for beneficial purposes while minimizing its risks. It’s not about being afraid of gamma radiation; it’s about understanding it and respecting its power.